Human Attachments Shape Interbrain Synchrony toward Efficient Performance of Social Goals
Abstract
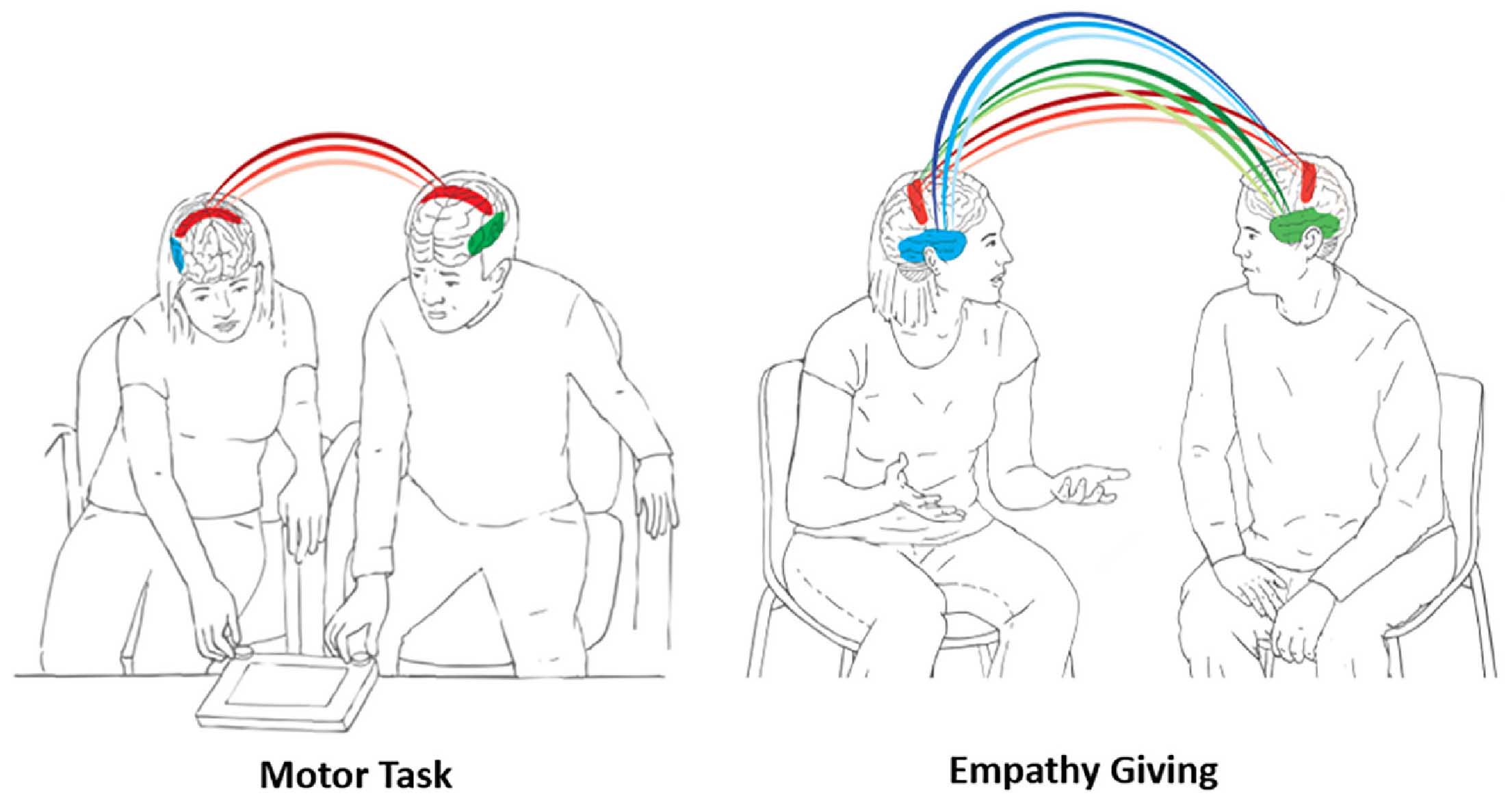
The human brain has undergone massive expansion across primate evolution through life amidst multi-layered social attachments; within families, among friends, and between clan members and this enabled humans to coordinate their brains with those of others toward the execution of complex social goals. We examined how human attachments facilitate efficient, resource-sensitive performance of social goals by balancing neural and behavioral synchrony. Using hyperscanning EEG, we collected neural data from male-female pairs in three groups (N=158, 79 pairs); long-term couples, best friends, and unfamiliar group members, during two ecologically-valid naturalistic tasks; motor coordination and empathy giving. Across groups and tasks, neural synchrony was supported by behavior coordination and orchestrated multiple neural rhythms. In the goal-directed motor task, interbrain synchrony implicated beta and gamma rhythms localized to sensorimotor areas. Couples showed the highest neural synchrony combined with greatest behavioral synchrony and such brain-behavior linkage resulted in speedy performance, conserving energy in the long run. The socially-oriented empathy task triggered neural synchrony in widely-distributed sensorimotor and bilateral temporal regions, integrated alpha, beta, and gamma rhythms, and implicated brain-behavior complementarity; couples displayed the highest behavioral synchrony combined with lowest neural synchrony toward greatest felt support while strangers exhibited the opposite pattern. Findings suggest that human attachments provide a familiar backdrop of temporal regularities, required for the brain’s allostatic function, and interbrain and behavioral synchrony are sculpted by familiarity and closeness toward resource-sensitive performance of survival-related social goals, toiled by two.
Highlights
- • We tested neural and behavior synchrony in male-female couples, best friends, and strangers
- • During motor coordination, beta and gamma synchrony emerged in central regions
- • Couples showed highest neural and behavioral synchrony and fastest performance
- • Empathy-giving elicited alpha, beta, and gamma synchrony in central and temporal areas
- • Couples showed highest behavior and lowest neural synchrony and strangers the reverse

Leave a Reply